
High Quality Molecular Sieve 3A
CAS NO.: 11113-61-4
Purity :98%
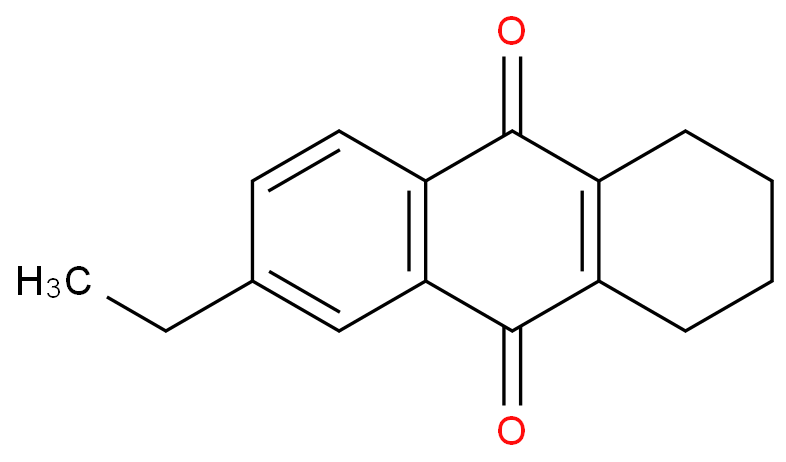
Tetrahydro-2-ethylanthraquinone
CAS NO.: 15547-17-8
Purity :98%
| Compound | Aluminum oxide | ||||
|---|---|---|---|---|---|
| CAS No. | 1344-28-1 | Catalog No. | 2023010202 | Brand | V-SK |
| Purity | 93% | Packing | 1ton | Grade | Molecular Biology Grade |
| Lead Time | 7Day (s) | Origin | ChinaShandongZibo | Loading Port | China,Shandong,Zibo |
| Boiling Point | 2980ºC |
|---|---|
| Stability | Stable under normal shipping and handling conditions. |
| Storage Condition | Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. |
| Appearance & Physical State | white odorless crystalline powder |
| Refractive Index | 1.765 |
| Density | 1.06 g/mL at 25ºC |
| Melting Point | 2050ºC |
Activated alumina carrier is a porous, highly dispersive solid material with a large surface area. Its microporous surface has the characteristics required by catalysis, such as adsorption performance, surface activity, excellent thermal stability, etc., so it is widely used as a catalyst and catalyst carrier for chemical reactions.
1. Activated alumina is used as catalyst carrier. In the catalytic reaction with simple function, activated alumina does not directly participate in the catalytic process, but dilutes, supports and disperses precious metals. More than 70% of activated alumina is used as catalyst support. In addition to the above functions, in some reactions, activated alumina also has the functions of enhancing thermal stability and mechanical stability. For example, pd/AL2O3, cu/r-AL2O3 used in automobile exhaust gas purification catalysts and catalysts for petroleum cracking reactions all belong to this type. Nickel catalyst used in olefin hydrogenation reaction is supported on activated alumina, Its thermal stability range is larger than that of the catalyst loaded with nickel diatomite.
2. Activated alumina is used as active catalyst. Activated alumina has obvious adsorbent characteristics, such as H-H bond, C-H bond, etc. Therefore, it can be directly added into the reaction system as active catalyst in hydrocarbon cracking, alcohol dehydration to ether and other reactions. For example, when ethanol is dehydrated to produce ethylene, there are both acidic and alkaline centers on the surface of activated alumina. Therefore, activated alumina itself is a good catalyst.