1.Identification
1.1 GHS Product identifier
| Product name | Formaldehyde |
|---|
1.2 Other means of identification
| Product number | - |
|---|---|
| Other names | superlysoform |
1.3 Recommended use of the chemical and restrictions on use
| Identified uses | For industry use only. Formaldehyde is used predominantly as a chemical intermediate. It also has minor uses in agriculture, as an analytical reagent, in concrete and plaster additives, cosmetics, disinfectants, fumigants, photography, and wood preservation. One of the most common uses of formaldehyde in the U.S is manufacturing urea-formaldehyde resins, used in particleboard products. Formaldehyde (as urea formaldehyde foam) was extensively used as an insulating material until 1982 when it was banned by the U.S. Consumer Product Safety Commission. |
|---|---|
| Uses advised against | no data available |
1.4 Supplier's details
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
1.5 Emergency phone number
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
2.Hazard identification
2.1 Classification of the substance or mixture
Acute toxicity - Oral, Category 3
Acute toxicity - Dermal, Category 3
Skin corrosion, Category 1B
Skin sensitization, Category 1
Acute toxicity - Inhalation, Category 3
Germ cell mutagenicity, Category 2
Carcinogenicity, Category 1B
2.2 GHS label elements, including precautionary statements
| Pictogram(s) | 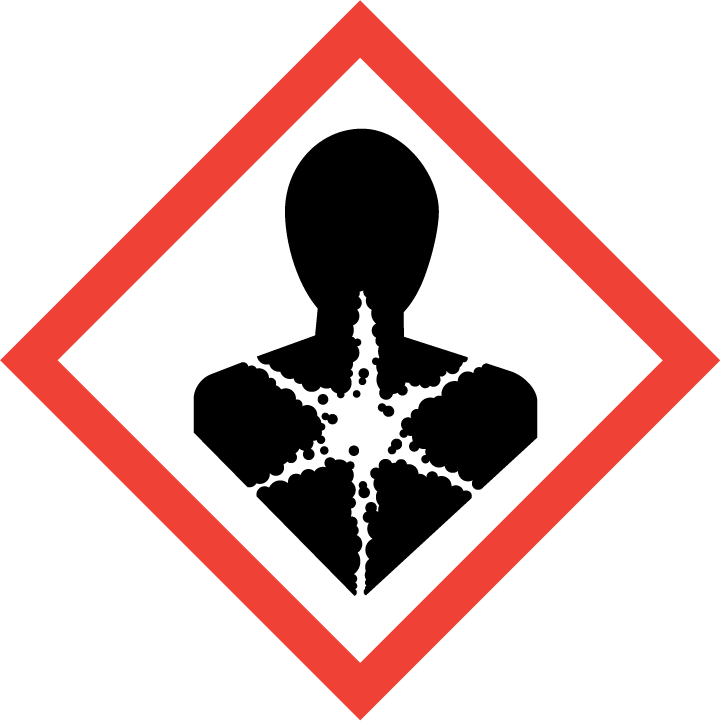 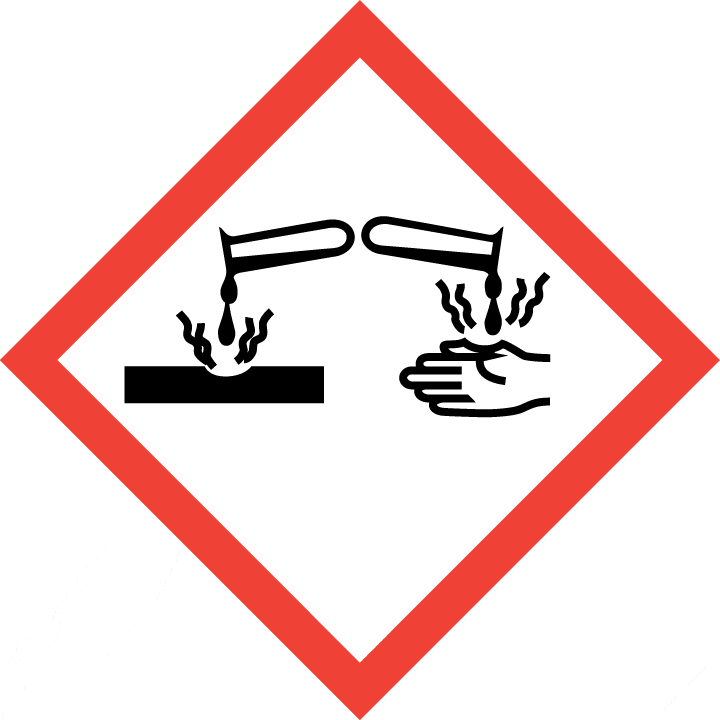  |
|---|---|
| Signal word | Danger |
| Hazard statement(s) | H301 Toxic if swallowed H311 Toxic in contact with skin H314 Causes severe skin burns and eye damage H317 May cause an allergic skin reaction H331 Toxic if inhaled H341 Suspected of causing genetic defects H350 May cause cancer |
| Precautionary statement(s) | |
| Prevention | P264 Wash ... thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P280 Wear protective gloves/protective clothing/eye protection/face protection. P260 Do not breathe dust/fume/gas/mist/vapours/spray. P261 Avoid breathing dust/fume/gas/mist/vapours/spray. P272 Contaminated work clothing should not be allowed out of the workplace. P271 Use only outdoors or in a well-ventilated area. P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. |
| Response | P301+P310 IF SWALLOWED: Immediately call a POISON CENTER/doctor/… P321 Specific treatment (see ... on this label). P330 Rinse mouth. P302+P352 IF ON SKIN: Wash with plenty of water/... P312 Call a POISON CENTER/doctor/…if you feel unwell. P361+P364 Take off immediately all contaminated clothing and wash it before reuse. P301+P330+P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353 IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water [or shower]. P363 Wash contaminated clothing before reuse. P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing. P310 Immediately call a POISON CENTER/doctor/… P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P333+P313 If skin irritation or rash occurs: Get medical advice/attention. P362+P364 Take off contaminated clothing and wash it before reuse. P311 Call a POISON CENTER/doctor/… P308+P313 IF exposed or concerned: Get medical advice/ attention. |
| Storage | P405 Store locked up. P403+P233 Store in a well-ventilated place. Keep container tightly closed. |
| Disposal | P501 Dispose of contents/container to ... |
2.3 Other hazards which do not result in classification
none
3.Composition/information on ingredients
3.1 Substances
| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| Formaldehyde | Formaldehyde | 50-00-0 | none | 100% |
4.First-aid measures
4.1 Description of necessary first-aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
Fresh air, rest. Half-upright position. Artificial respiration may be needed. Refer immediately for medical attention.
In case of skin contact
Remove contaminated clothes. Rinse skin with plenty of water or shower. Seek medical attention if you feel unwell.
In case of eye contact
Rinse with plenty of water (remove contact lenses if easily possible). Refer immediately for medical attention.
If swallowed
Rinse mouth. Do NOT induce vomiting. Refer immediately for medical attention.
4.2 Most important symptoms/effects, acute and delayed
Exposure Routes: inhalation, skin and/or eye contact Symptoms: Irritation eyes, nose, throat, respiratory system; lacrimation (discharge of tears); cough; wheezing; [potential occupational carcinogen] Target Organs: Eyes, respiratory system (NIOSH, 2016)
The probable oral lethal dose for humans is 0.5-5 g/kg, or between 1 ounce and 1 pint for a 150 pound person. Acute -- below 1 ppm, odor perceptible to most. 2-3 ppm, mild tingling of eyes. 4-5 ppm, increased discomfort, mild lacrimation. 10 ppm, profuse lacrimation; can be withstood only for few minutes. 10-20 ppm, breathing difficult, cough, severe burning of nose and throat. 50-100 ppm, acute irritation of respiratory tract, very serious injury likely. Skin -- primary irritation from strong solutions, gas. Delayed -- sensitization dermatitis. Suspected carcinogen. Effects in women include menstrual disorders and secondary sterility. Solutions splashed in eyes have caused injuries ranging from severe, permanent corneal opacification and loss of vision to minor discomfort. In people sensitized to formaldehyde, late asthmatic reactions may be provoked by brief exposures at approximately 3 ppm. (EPA, 1998)
Excerpt from ERG Guide 132 [Flammable Liquids - Corrosive]: May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. (ERG, 2016)
Vapor or dust irritates eyes, mucous membranes, and skin; may cause dermatitis. Ingestion of solid or of a solution in water irritates mouth, throat, and stomach and may cause death. (USCG, 1999)
4.3 Indication of immediate medical attention and special treatment needed, if necessary
Emergency and supportive measures: 1. Maintain open airway and assist ventilation if necessary. 2. Inhalation. Treat bronchospasm and pulmonary edema if they occur. Administer supplemental oxygen, and observe for at least 4 to 6 hours. 3. Ingestion. a. Treat coma and shock if they occur. b. Administer intravenous saline or other crystalloids to replace fluid losses caused by gastroenteritis. Avoid fluid overload in patients with inhalation exposure because of the risk of pulmonary edema. c. Treat metabolic acidosis with sodium bicarbonate.
5.Fire-fighting measures
5.1 Extinguishing media
Suitable extinguishing media
Use water spray, dry chemical, alcohol foam, or carbon dioxide. Use water to keep fire exposed containers cool. If leak or spill has not ignited, use water spray to disperse vapors, and to protect men attempting to stop leak. Water spray may be used to flush spills away from exposures and to dilute spills to nonflammable mixtures.
5.2 Specific hazards arising from the chemical
Excerpt from ERG Guide 171 [Substances (Low to Moderate Hazard)]: Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot. For UN3508, be aware of possible short circuiting as this product is transported in a charged state. (ERG, 2016)
Toxic vapors such as carbon dioxide and carbon monoxide are generated during combustion. Explosion hazard: when aqueous formaldehyde solutions are heated above their flash points, a potential for explosion hazard exists. High formaldehyde concentration or methanol content lowers flash point. Reacts with nitrogen oxides at about 180; the reaction becomes explosive. Also reacts violently with perchloric acid-aniline, performic acid, nitromethane, magnesium carbonate, and hydrogen peroxide. When heated, irritant formaldehyde gas evolved from solution. The main products of decomposition are carbon monoxide and hydrogen. Metals such as platinum, copper, chromia, and alumina also catalyze the formation of methanol, methylformate, formic acid, carbon dioxide, and methane. Reacts with peroxide, nitrogen oxide, and performic acid causing explosions. Can react with hydrogen chloride or other inorganic chlorides to form bis-chloromethylether (BCME), a known carcinogen. Very reactive, combines readily with many substances, 40% solution is powerful reducing agent. Incompatible with amines, azo compounds, dithiocarbamates, alkali and alkaline earth metals, nitrides, nitro compounds, unsaturated aliphatics and sulfides, organic peroxides, oxidizing agents, and reducing agents. Aqueous solutions are unstable. Commercial formaldehyde-alcohol solutions are stable. Gas is stable in absence of water. Avoid oxidizing and alkaline materials. Hazardous polymerization may occur. Compound will polymerize with active organic materials such as phenol. Will polymerize violently in the presence of caustics and nitrides; (amines) exothermic reaction, (Azo compound) exothermic reaction giving off nitrogen gas, (caustics) heat generation and violent polymerization, (dithiocarbamates) formation of flammable gases and toxic fumes, formation of carbon disulfide may result, (alkali and alkaline earth metals) heat generation and formation of a flammable hydrogen gas. (EPA, 1998)
Excerpt from ERG Guide 132 [Flammable Liquids - Corrosive]: Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Those substances designated with a (P) may polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. (ERG, 2016)
Behavior in Fire: Changes to formaldehyde gas, which is highly flammable. (USCG, 1999)
5.3 Special protective actions for fire-fighters
Wear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures
6.1 Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautions
Evacuate danger area! Consult an expert! Personal protection: gas-tight chemical protection suit including self-contained breathing apparatus. Remove all ignition sources. Turn off gas at source if possible. Remove gas with fine water spray.
6.3 Methods and materials for containment and cleaning up
Use fluorocarbon water spray, Cellosize and Hycar to diminish vapors. Sodium carbonate, ammonium hydroxide, or sodium sulfite can neutralize the spill.
7.Handling and storage
7.1 Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Fireproof. Cool. Separated from incompatible materials. See Chemical Dangers.... Minimum storage temperature to prevent polymerization range from 83 deg F for 37% formaldehyde containing 0.05% methyl alcohol to 29 deg F for formaldehyde containing 15% methyl alcohol.
8.Exposure controls/personal protection
8.1 Control parameters
Occupational Exposure limit values
Recommended Exposure Limit: 10 Hour Time-Weighted Average: 0.016 ppm. /Formaldehyde/ /Formalin (as formaldehyde)/
Recommended Exposure Limit: 15 Minute Ceiling Value: 0.1 ppm. /Formaldehyde/ /Formalin (as formaldehyde)/
NIOSH usually recommends that occupational exposures to carcinogens be limited to the lowest feasible concentration. /Formaldehyde/ /Formalin (as formaldehyde)/
Biological limit values
no data available
8.2 Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE)
Eye/face protection
Safety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Wear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protection
Wear dust mask when handling large quantities.
Thermal hazards
no data available
9.Physical and chemical properties
| Physical state | Colorless gas |
|---|---|
| Colour | Nearly colorless gas [Note: Often used in an aqueous solution]. /Pure formaldehyde/ |
| Odour | Pungent, suffocating odor |
| Melting point/ freezing point | 250°C(dec.)(lit.) |
| Boiling point or initial boiling point and boiling range | 98°C(lit.) |
| Flammability | Flammable GasExtremely flammable. |
| Lower and upper explosion limit / flammability limit | Lower flammable limit: 7.0% by volume; Upper flammable limit: 73% by volume |
| Flash point | 64°C |
| Auto-ignition temperature | 300°C |
| Decomposition temperature | 120-180°C |
| pH | pH: 2.8 to 4.0 /Formaldehyde soln/ |
| Kinematic viscosity | 0.1421 cP at 25°C |
| Solubility | In water:soluble, > 100 g/100 ml(20 °C) |
| Partition coefficient n-octanol/water (log value) | log Kow = 0.35 |
| Vapour pressure | >1 atm (20 °C) |
| Density and/or relative density | 1.09g/mLat 20°C |
| Relative vapour density | 1.03 (vs air) |
| Particle characteristics | no data available |
10.Stability and reactivity
10.1 Reactivity
no data available
10.2 Chemical stability
On standing, especially in the cold, may become cloudy, and on exposure to very low temperature ppt of trioxymethylene formed; in air it slowly oxidizes to formic acid /40% solution/.
10.3 Possibility of hazardous reactions
Flammable liquid when exposed to heat or flame; can react vigorously with oxidizers. ... The gas is a more dangerous fire hazard than the vapor.The gas mixes well with air, explosive mixtures are easily formed.Dust explosion possible if in powder or granular form, mixed with air. If dry, it can be charged electrostatically by swirling, pneumatic transport, pouring, etc.FORMALDEHYDE (ENVIRONMENTALLY HAZARDOUS SUBSTANCES, SOLID, N.O.S.) may react violently with strong oxidizing agents (hydrogen peroxide, performic acid, perchloric acid in the presence of aniline, potassium permanganate, nitromethane). May react with bases (sodium hydroxide, potassium hydroxide, ammonia), and with nitrogen dioxide (explosive reaction around 180°C). May react with hydrochloric acid to form highly toxic bis(chloromethyl) ether. Polymerization reaction with phenol may develop sudden destructive pressure [Bretherick, 5th ed., 1995, p.168]. May generate flammable and/or toxic gases in combination with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. May generate toxic formaldehyde gas when heated. Can react with air to give first peroxo acids, and ultimately formic acid. These reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). Incompatible with liquid oxygen.
10.4 Conditions to avoid
no data available
10.5 Incompatible materials
(Amines) exothermic reaction, (AZO cmpd) exothermic reaction giving off nitrogen gas, (caustics) heat generation and violent polymerization, (dithiocarbamates) formation of flammable gasses and toxic fumes, formation of carbon disulfide may result, (alkali and alkaline earth metals) heat generation and formation of flammable hydrogen gas, (nitrides) heat generation, formation of flammable ammonia gas and violent polymerization, (nitro compd) heat generation, (unsaturated aliphatics and sulfides) heat generation, (organic peroxides) violent reaction, (oxidizing agents) heat generation, fire, and decomposition, (reducing agents) heat generation and formation of flammable gasses. /From table/
10.6 Hazardous decomposition products
Uncatalyzed decomposition is very slow below 300°C; extrapolation of kinetic data to 400°C indicates that the rate of decomposition is about 0.44%/min at 101 kPa (1 atm). The main products are carbon monoxide and hydrogen. Metals such as platinium, copper, chromia, and alumina also catalyze the formation of methanol, methylformate, formic acid, carbon dioxide, and methane.
11.Toxicological information
Acute toxicity
- Oral: LD50 Rat oral 100 mg/kg /SRP: percent solution not specified/
- Inhalation: LC50 Rat inhalation 0.82 mg/L (1/2 hour)
- Dermal: LD50 Rabbit percutaneous 270 mg/kg /Formalin/
Skin corrosion/irritation
no data available
Serious eye damage/irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
There is sufficient evidence in humans for the carcinogenicity of formaldehyde. Formaldehyde causes cancer of the nasopharynx and leukaemia. Also, a positive association has been observed between exposure to formaldehyde and sinonasal cancer. There is sufficient evidence in experimental animals for the carcinogenicity of formaldehyde. The Working Group was not in full agreement on the evaluation of formaldehyde causing leukaemias in humans, with a small majority viewing the evidence as sufficient of carcinogenicity and the minority viewing the evidence as limited. Particularly relevant to the discussions regarding sufficient evidence was a recent study accepted for publication which, for the first time, reported aneuploidy in blood of exposed workers characteristic of myeloid leukaemia and myelodysplastic syndromes, with supporting information suggesting a decrease in the major circulating blood-cell types and in circulating haematological precursor cells. The authors and Working Group felt that this study needed to be replicated. Formaldehyde is carcinogenic to humans (Group 1).
Reproductive toxicity
An increased incidence of menstrual disorders were observed in female workers using urea-formaldehyde resins. However, possible confounding factors were not evaluated in this study. A study of hospital equipment sterilizing workers did not report an association between formaldehyde exposure and increased spontaneous abortions. Developmental effects, such as birth defects, have not been observed in animal studies with formaldehyde.
STOT-single exposure
no data available
STOT-repeated exposure
no data available
Aspiration hazard
no data available
12.Ecological information
12.1 Toxicity
- Toxicity to fish: LC50; Species: Oncorhynchus mykiss (Rainbow trout) weight 0.63 g; Conditions: static; Concentration: 118 ppm for 96 hr (95% confidence limit: 99.7-140 ppm) /37% AI formulated product
- Toxicity to daphnia and other aquatic invertebrates: EC50; Species: Daphnia magna (Water Flea) age <24 hr; Conditions: freshwater, static; Concentration: 14600 ug/L for 48 hr (95% confidence interval: 11300-18000 ug/L); Effect: intoxication, immobilization /37% purity
- Toxicity to algae: EC50; Species: Scenedesmus subspicatus (Green Algae); Conditions: freshwater, static; Concentration: 211000 ug/L for 49-79 min; Effect: population, decreased photosynthesis /formulation
- Toxicity to microorganisms: no data available
12.2 Persistence and degradability
AEROBIC: Formaldehyde, present at 100 mg/L, reached 91% of its theoretical BOD in 2 weeks using an activated sludge inoculum at 30 mg/L in the Japanese MITI test which classified the compound as readily biodegradable(1). Using OECD Guide-line 301D (Ready Biodegradability: Closed Bottle Test), formaldehyde achieved 90% in 28 days using non-acclimated inoculum which classified the compound as readily biodegradable(2). Formaldehyde in aqueous effluent was degraded by activated sludge and sewage in 48-72 hr(3-6). In a die-away test using water from a stagnant lake, degradation was complete in 30 hours under aerobic conditions(6). Other biodegradation screening tests gave half-lives ranging from <1 to 17.3 days(7-12).
12.3 Bioaccumulative potential
An estimated BCF of 3 was calculated for formaldehyde(SRC), using a log Kow of 0.35(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low(SRC). Experiments performed on a variety of fish and shrimp showed no bioconcentration of formaldehyde(4,5).
12.4 Mobility in soil
The Koc of formaldehyde is estimated as 8(SRC), using a log Kow of 0.35(1) and a regression-derived equation(2). According to a classification scheme(3), this estimated Koc value suggests that formaldehyde is expected to have very high mobility in soil(SRC). Formaldehyde gas adsorbs on clay minerals to a degree at high gas concentrations which is an important quality in its use as a soil fumigant(4). In addition, formaldehyde may interact with humic substances in soil(5) resulting in decreased mobility.
12.5 Other adverse effects
no data available
13.Disposal considerations
13.1 Disposal methods
Product
The material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packaging
Containers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information
14.1 UN Number
| ADR/RID: UN2209 | IMDG: UN2209 | IATA: UN2209 |
14.2 UN Proper Shipping Name
| ADR/RID: FORMALDEHYDE SOLUTION with not less than 25% formaldehyde |
| IMDG: FORMALDEHYDE SOLUTION with not less than 25% formaldehyde |
| IATA: FORMALDEHYDE SOLUTION with not less than 25% formaldehyde |
14.3 Transport hazard class(es)
| ADR/RID: 8 | IMDG: 8 | IATA: 8 |
14.4 Packing group, if applicable
| ADR/RID: III | IMDG: III | IATA: III |
14.5 Environmental hazards
| ADR/RID: no | IMDG: no | IATA: no |
14.6 Special precautions for user
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Code
no data available
15.Regulatory information
15.1 Safety, health and environmental regulations specific for the product in question
| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| Formaldehyde | Formaldehyde | 50-00-0 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
16.Other information
Information on revision
| Creation Date | Aug 11, 2017 |
|---|---|
| Revision Date | Aug 11, 2017 |
Abbreviations and acronyms
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
References
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/






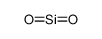
-
-
-
-
-
-
-
-
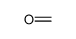
-
-
-
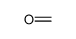
-
-
-

-
-
-
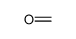
-
-
-
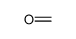
-
-
-

-
-
-

-
-
-
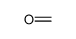
-
More Suppliers>>Baoji Guokang Bio-Technology Co., Ltd
CHINA
Purity: 38%
Lead Time: 7 Day(s)
Price: -
Wenzhou Win-Win Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 3 Day(s)
Price: -
Nanjing Yushan Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 3 Day(s)
Price: -
Hangzhou J&H Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Xiamen Zhixin Chemical Co., Ltd.
CHINA
Purity: 99%
Lead Time: 3 Day(s)
Price: -
Skyrun Industrial Co., Limited
CHINA
Purity: 99%
Lead Time: 7 Day(s)
Price: -
Henan Coreychem Co.,Ltd
CHINA
Purity: 98%
Lead Time: 3 Day(s)
Price: Min $1 /g
CHINA
Purity: 99%
Lead Time: 7 Day(s)
Price: Min $200 /吨
ShiJiaZhuang Xinlongwei Chemical Co.,Ltd
CHINA
Purity: 37%
Lead Time: 14 Day(s)
Price: Min $1401 /吨
CHINA
Purity: 99%
Lead Time: 3 Day(s)
Price: Min $100 /kg