1.Identification
1.1 GHS Product identifier
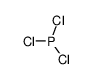
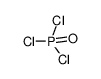
| Product name | phosphorus trichloride |
|---|
1.2 Other means of identification
| Product number | - |
|---|---|
| Other names | Phosphorous trichloride |
1.3 Recommended use of the chemical and restrictions on use
| Identified uses | For industry use only. Intermediates,Process regulators |
|---|---|
| Uses advised against | no data available |
1.4 Supplier's details
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
1.5 Emergency phone number
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
2.Hazard identification
2.1 Classification of the substance or mixture
Acute toxicity - Oral, Category 2
Skin corrosion, Category 1A
Acute toxicity - Inhalation, Category 2
Specific target organ toxicity – repeated exposure, Category 2
2.2 GHS label elements, including precautionary statements
| Pictogram(s) |    |
|---|---|
| Signal word | Danger |
| Hazard statement(s) | H300 Fatal if swallowed H314 Causes severe skin burns and eye damage H330 Fatal if inhaled H373 May cause damage to organs through prolonged or repeated exposure |
| Precautionary statement(s) | |
| Prevention | P264 Wash ... thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P260 Do not breathe dust/fume/gas/mist/vapours/spray. P280 Wear protective gloves/protective clothing/eye protection/face protection. P271 Use only outdoors or in a well-ventilated area. P284 [In case of inadequate ventilation] wear respiratory protection. |
| Response | P301+P310 IF SWALLOWED: Immediately call a POISON CENTER/doctor/… P321 Specific treatment (see ... on this label). P330 Rinse mouth. P301+P330+P331 IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353 IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water [or shower]. P363 Wash contaminated clothing before reuse. P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing. P310 Immediately call a POISON CENTER/doctor/… P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P320 Specific treatment is urgent (see ... on this label). P314 Get medical advice/attention if you feel unwell. |
| Storage | P405 Store locked up. P403+P233 Store in a well-ventilated place. Keep container tightly closed. |
| Disposal | P501 Dispose of contents/container to ... |
2.3 Other hazards which do not result in classification
none
3.Composition/information on ingredients
3.1 Substances
| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| phosphorus trichloride | phosphorus trichloride | 7719-12-2 | none | 100% |
4.First-aid measures
4.1 Description of necessary first-aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
Fresh air, rest. Half-upright position. Artificial respiration may be needed. Refer for medical attention.
In case of skin contact
Remove contaminated clothes. Rinse skin with plenty of water or shower. Refer for medical attention .
In case of eye contact
First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowed
Rinse mouth. Do NOT induce vomiting. Refer for medical attention .
4.2 Most important symptoms/effects, acute and delayed
This material is highly toxic; it may cause death or permanent injury. Contact is highly irritating to the skin, eyes, and mucous membranes, and the material is an irritant through oral and inhalation exposure. (EPA, 1998)
4.3 Indication of immediate medical attention and special treatment needed, if necessary
Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 ml/kg up to 200 ml of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Chlorine and related compounds/
5.Fire-fighting measures
5.1 Extinguishing media
Suitable extinguishing media
Evacuation: If fire becomes uncontrollable or container is exposed to direct flame - consider evacuation of one-third (1/3) mile radius.
5.2 Specific hazards arising from the chemical
This material will react violently with water, producing heat and toxic and corrosive fumes. When heated to decomposition, it emits highly toxic fumes of chlorides and phosphorus oxides. It may ignite other combustible materials. Reacts violently with water. Reacts explosively with acetic acid, aluminum, chromyl chloride, diallylphosphite and allyl alcohol, dimethyl sulfoxide, fluorine, hydroxylamine, iodine monochloride, lead dioxide, nitric acid, nitrous acid, organic matter, potassium, and sodium. Avoid contact with water, steam, or acids. Hazardous polymerization may not occur. (EPA, 1998)
5.3 Special protective actions for fire-fighters
Wear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures
6.1 Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautions
Evacuate danger area! Consult an expert! Ventilation. Collect leaking and spilled liquid in sealable containers as far as possible. Absorb remaining liquid in dry sand or inert absorbent. Then store and dispose of according to local regulations. Personal protection: chemical protection suit including self-contained breathing apparatus.
6.3 Methods and materials for containment and cleaning up
Environmental considerations: water spill: Neutralize with agricultural lime (CaO), crushed limestone (CaCO3), or sodium bicarbonate (NaCO3). Use mechanical dredges or lifts to remove immobilized masses of pollutant and precipitates. Adjust pH to neutral (pH=7).
7.Handling and storage
7.1 Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Provision to contain effluent from fire extinguishing. Separated from food and feedstuffs. See Chemical Dangers. Dry. Well closed. Ventilation along the floor.Provision to contain effluent from fire extinguishing. Separated from food and feedstuffs. See Chemical Dangers. Dry. Well closed. Ventilation along the floor.
8.Exposure controls/personal protection
8.1 Control parameters
Occupational Exposure limit values
Recommended Exposure Limit: 10 Hr Time-Weighted Avg: 0.2 ppm (1.5 mg/cu m).
Recommended Exposure Limit: 15 Min Short-Term Exposure Limit: 0.5 ppm (3 mg/cu m).
Biological limit values
no data available
8.2 Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE)
Eye/face protection
Safety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Wear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protection
Wear dust mask when handling large quantities.
Thermal hazards
no data available
9.Physical and chemical properties
| Physical state | Clear liquid |
|---|---|
| Colour | Clear colorless, fuming liquid |
| Odour | Pungent |
| Melting point/ freezing point | -112ºC |
| Boiling point or initial boiling point and boiling range | 76ºC |
| Flammability | Noncombustible Liquid; however, a strong oxidizer that may ignite combustibles upon contact.Not combustible. Many reactions may cause fire or explosion. Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | no data available |
| Flash point | none |
| Auto-ignition temperature | Not flammable (USCG, 1999) |
| Decomposition temperature | no data available |
| pH | no data available |
| Kinematic viscosity | 0.65 cP at 0°C; 0.438 cP at 50°C |
| Solubility | In water:reacts |
| Partition coefficient n-octanol/water (log value) | no data available |
| Vapour pressure | 23.32 psi ( 55 °C) |
| Density and/or relative density | 1.363g/mLat 25°C |
| Relative vapour density | 4.75 (vs air) |
| Particle characteristics | no data available |
10.Stability and reactivity
10.1 Reactivity
no data available
10.2 Chemical stability
Stable under recommended storage conditions.
10.3 Possibility of hazardous reactions
Reacts with water to form hydrochloric acid, which reacts with most metals to form flammable hydrogen gas.The vapour is heavier than air.PHOSPHORUS TRICHLORIDE is a strong reducing agent that may ignite combustible organic materials upon contact. May generate flammable and potentially explosive gaseous hydrogen upon contact with many common metals (except nickel and lead). Reactions with water are violent and produce heat and flashes of fire. Gives intensely exothermic reactions with iodine monochloride [Mellor 2, Supp. 1:502. 1956]. Several laboratory explosions have been reported arising from mixtures with acetic acid, along with other acids, sulfuric acid and derivatives, carboxylic acids, etc. These have been ascribed to poor heat control allowing the formation of phosphine [J. Am. Chem. Soc. 60:488. 1938]. Ignites when mixed with hydroxylamine [Mellor 8:290. 1946-47]. Causes an explosion on contact with nitric acid [Comp. Rend. 28:86]. It is incompatible with many common oxidants such as: sodium peroxide, fluorine, chromyl chloride, iodine chloride, to name a few. Isopropanol can react with PCl3, forming toxic HCl gas. (Logsdon, John E., Richard A. Loke., Isopropyl Alcohol. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 1996.)
10.4 Conditions to avoid
no data available
10.5 Incompatible materials
Reacts with water to form hydrochloric acid, which reacts with most metals to form flammable hydrogen gas.
10.6 Hazardous decomposition products
When heated to decomposition it emits toxic fumes of /hydrogen chloride and phosphorus oxide/.
11.Toxicological information
Acute toxicity
- Oral: LD50 Rat oral 550 mg/kg
- Inhalation: LC50 Rat inhalation 104 ppm/4 hr
- Dermal: no data available
Skin corrosion/irritation
no data available
Serious eye damage/irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
no data available
Reproductive toxicity
no data available
STOT-single exposure
no data available
STOT-repeated exposure
no data available
Aspiration hazard
no data available
12.Ecological information
12.1 Toxicity
- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
12.2 Persistence and degradability
no data available
12.3 Bioaccumulative potential
no data available
12.4 Mobility in soil
no data available
12.5 Other adverse effects
no data available
13.Disposal considerations
13.1 Disposal methods
Product
The material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packaging
Containers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information
14.1 UN Number
| ADR/RID: UN3390 | IMDG: UN3390 | IATA: UN3390 |
14.2 UN Proper Shipping Name
| ADR/RID: TOXIC BY INHALATION LIQUID, CORROSIVE, N.O.S. with an LC50 lower than or equal to 1000 ml/m3 and saturated vapour concentration greater than or equal to 10 LC50 |
| IMDG: TOXIC BY INHALATION LIQUID, CORROSIVE, N.O.S. with an LC50 lower than or equal to 1000 ml/m3 and saturated vapour concentration greater than or equal to 10 LC50 |
| IATA: TOXIC BY INHALATION LIQUID, CORROSIVE, N.O.S. with an LC50 lower than or equal to 1000 ml/m3 and saturated vapour concentration greater than or equal to 10 LC50 |
14.3 Transport hazard class(es)
| ADR/RID: 8 | IMDG: 8 | IATA: 8 |
14.4 Packing group, if applicable
| ADR/RID: I | IMDG: I | IATA: I |
14.5 Environmental hazards
| ADR/RID: no | IMDG: no | IATA: no |
14.6 Special precautions for user
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Code
no data available
15.Regulatory information
15.1 Safety, health and environmental regulations specific for the product in question
| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| phosphorus trichloride | phosphorus trichloride | 7719-12-2 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Not Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
16.Other information
Information on revision
| Creation Date | Aug 16, 2017 |
|---|---|
| Revision Date | Aug 16, 2017 |
Abbreviations and acronyms
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
References
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/




-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
More Suppliers>>Hangzhou J&H Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 2 Day(s)
Price: -
Hangzhou J&H Chemical Co., Ltd.
CHINA
Purity: >97%
Lead Time: 7 Day(s)
Price: -
Hangzhou Bingochem Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
CHINA
Purity: 99%
Lead Time: 3 Day(s)
Price: Min $100 /桶
Panjin Yanfeng Technology Co., Ltd.
CHINA
Purity: -%
Lead Time: 7 Day(s)
Price: -
Xinji Hongzheng Chemical Co., Ltd.
CHINA
Purity: 99%
Lead Time: 14 Day(s)
Price: -
Beijing Coupling Technology Co., Ltd.
CHINA
Purity: 97%
Lead Time: 7 Day(s)
Price: Min $141.67 /g
Shanghai Runjie Chemical Reagent Co., Ltd.
CHINA
Purity: AR%
Lead Time: 3 Day(s)
Price: Min $48 /ml
Shen zhen nanganghengshun trading co.ltd.,
CHINA
Purity: 99%
Lead Time: 7 Day(s)
Price: -
Chengdu Changzheng Glass Co.,Ltd.
CHINA
Purity: 96%
Lead Time: 7 Day(s)
Price: -