1.Identification
1.1 GHS Product identifier
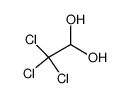
| Product name | chloral hydrate |
|---|
1.2 Other means of identification
| Product number | - |
|---|---|
| Other names | Trichloroacetaldehyde Hydrate |
1.3 Recommended use of the chemical and restrictions on use
| Identified uses | For industry use only. |
|---|---|
| Uses advised against | no data available |
1.4 Supplier's details
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
1.5 Emergency phone number
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
2.Hazard identification
2.1 Classification of the substance or mixture
Acute toxicity - Oral, Category 3
Skin irritation, Category 2
Eye irritation, Category 2
2.2 GHS label elements, including precautionary statements
| Pictogram(s) |  |
|---|---|
| Signal word | Danger |
| Hazard statement(s) | H301 Toxic if swallowed H315 Causes skin irritation H319 Causes serious eye irritation |
| Precautionary statement(s) | |
| Prevention | P264 Wash ... thoroughly after handling. P270 Do not eat, drink or smoke when using this product. P280 Wear protective gloves/protective clothing/eye protection/face protection. |
| Response | P301+P310 IF SWALLOWED: Immediately call a POISON CENTER/doctor/… P321 Specific treatment (see ... on this label). P330 Rinse mouth. P302+P352 IF ON SKIN: Wash with plenty of water/... P332+P313 If skin irritation occurs: Get medical advice/attention. P362+P364 Take off contaminated clothing and wash it before reuse. P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P337+P313 If eye irritation persists: Get medical advice/attention. |
| Storage | P405 Store locked up. |
| Disposal | P501 Dispose of contents/container to ... |
2.3 Other hazards which do not result in classification
none
3.Composition/information on ingredients
3.1 Substances
| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| chloral hydrate | chloral hydrate | 302-17-0 | none | 100% |
4.First-aid measures
4.1 Description of necessary first-aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
Fresh air, rest. Artificial respiration may be needed. Refer immediately for medical attention.
In case of skin contact
Rinse skin with plenty of water or shower.
In case of eye contact
First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowed
Rinse mouth. Do NOT induce vomiting. Refer immediately for medical attention.
4.2 Most important symptoms/effects, acute and delayed
SYMPTOMS: Symptoms of exposure to this compound include general anesthesia, cardiac arrythmias and blood pressure depression. Other symptoms include hemorrhagic gastritis, enteritis, central nervous system depression, coma, severe respiratory depression, ventricular tachycardia and cardiac arrest. Dermatitis, swelling of the lids, hyperemia, edema of the conjuctiva and a sensation of irritation and tearing may also occur. Exposure may result in gastric irritation, rapid circulatory collapse, kidney and liver damage, heart damage, psychosis and leukopenia. Flatulence, abdominal distension, nausea, headache, giddiness, rashes and blood dyscrasias can occur. ACUTE/CHRONIC HAZARDS: This compound is an irritant of the skin and eyes. When heated to decomposition it emits toxic fumes of chlorine.
4.3 Indication of immediate medical attention and special treatment needed, if necessary
Maintain an open airway and assist ventilation if necessary. Administer supplemental oxygen. Treat coma, hypothermia, hypotension, and pulmonary edema if they occur. Monitor patients for at least 6 hours after ingestion, because delayed absorption may occur. Patients with chloral hydrate ingestion should be monitored 18 to 24 hours because of the risk of cardiac arrhythmias. Tachyarrhythmias caused by myocardial sensitization may be treated with propranolol or esmolol. ... Administer activated charcoal orally if conditions are appropriate. Gastric lavage is not necessary after small to moderate ingestions if activated charcoal can be given promptly. ... /Sedative-Hypnotic Agents/
5.Fire-fighting measures
5.1 Extinguishing media
Suitable extinguishing media
Fires involving this material can be controlled with a dry chemical, carbon dioxide or Halon extinguisher.
5.2 Specific hazards arising from the chemical
Flash point data for this chemical are not available; however, it is probably combustible.
5.3 Special protective actions for fire-fighters
Wear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures
6.1 Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautions
Personal protection: particulate filter respirator adapted to the airborne concentration of the substance. Sweep spilled substance into covered containers. If appropriate, moisten first to prevent dusting. Carefully collect remainder. Then store and dispose of according to local regulations.
6.3 Methods and materials for containment and cleaning up
Pick up and arrange disposal. Sweep up and shovel. Keep in suitable, closed containers for disposal.
7.Handling and storage
7.1 Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Separated from strong bases and food and feedstuffs.Chloral hydrate oral solution should be stored in tight, light-resistant containers. Chloral hydrate capsules should be stored at 15-30°C.
8.Exposure controls/personal protection
8.1 Control parameters
Occupational Exposure limit values
no data available
Biological limit values
no data available
8.2 Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE)
Eye/face protection
Safety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Wear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protection
Wear dust mask when handling large quantities.
Thermal hazards
no data available
9.Physical and chemical properties
| Physical state | white crystalline powde |
|---|---|
| Colour | COLORLESS OR WHITE CRYSTALS |
| Odour | Aromatic, penetrating and slightly acrid odor |
| Melting point/ freezing point | 19°C(lit.) |
| Boiling point or initial boiling point and boiling range | 57°C(lit.) |
| Flammability | Not combustible. Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | no data available |
| Flash point | -16°C(lit.) |
| Auto-ignition temperature | no data available |
| Decomposition temperature | 97°C |
| pH | 3.5-4.4 (10% soln in water) |
| Kinematic viscosity | no data available |
| Solubility | In water:660 g/100 mL |
| Partition coefficient n-octanol/water (log value) | no data available |
| Vapour pressure | 5 mm Hg at 10°C ; 10 mm Hg at 19.5°C; 60 mm Hg at 46.22°C |
| Density and/or relative density | 1.908 |
| Relative vapour density | 5.1 (Air= 1) |
| Particle characteristics | no data available |
10.Stability and reactivity
10.1 Reactivity
no data available
10.2 Chemical stability
SLOWLY VOLATILIZES ON EXPOSURE TO AIR.
10.3 Possibility of hazardous reactions
Combustible when exposed to heat or flame.CHLORAL HYDRATE is incompatible with alkalis, alkaline earth metals, alkali carbonates and soluble barbiturates. It is decomposed by sodium hydroxide. It reduces ammoniacal silver nitrate. It liquefies when triturated with an equal quantity of menthol, camphor or thymol. . Reaction of chloral hydrate with hydroxylamine produces toxic hydrogen cyanide gas, Org. Synth., 1941, Vol. 1, 377.
10.4 Conditions to avoid
no data available
10.5 Incompatible materials
no data available
10.6 Hazardous decomposition products
When heated to decomposition it emits toxic fumes of /hydrogen chloride/.
11.Toxicological information
Acute toxicity
- Oral: LD50 Rat oral 200-500 mg/kg
- Inhalation: no data available
- Dermal: no data available
Skin corrosion/irritation
no data available
Serious eye damage/irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
WEIGHT-OF-EVIDENCE CHARACTERIZATION: Under the 1986 cancer guidelines (EPA), chloral hydrate is assigned to Group C, possible human carcinogen. Under the 1996 proposed guidelines (EPA) for carcinogen risk assessment, chloral hydrate shows suggestive evidence of human carcinogenicity by the oral route of exposure. There are no carcinogenicity data from humans. Two bioassays in rats in which chloral hydrate was administered by drinking water show no increase in tumors at any site. Because only minimal toxicity was observed in the livers of the rats in these bioassays, the tests were not conducted at the maximum tolerated dose. A chronic bioassay in female mice showed a slight increase in the severity grade of hyperplasia and a slight increase in the incidence of adenoma in the pituatary gland pars distalis at the highest exposure tested. There is some evidence that chloral hydrate causes hepatocellular tumors in male mice. An earlier study showing an increase of hepatocellular adenomas or trabecular carcinomas following a single bolus exposure could not be confirmed in a study using more animals and higher exposures. Three separate 2-year bioassays in male mice show an increased incidence of hepatocellular adenoma or carcinoma. There are no data identifying a lesion that is a precursor to the hepatocellular tumors. The strain of mice used has a very high spontaneous incidence of hepatocellular tumors. Two of the matabolites of chloral hydrate, trichloroacetic acid and dichloroacetic acid, have been shown to cause hepatocellular tumors in rodents. Trichloroacetic acid causes hepatocellular tumors only in mice. Dichloroacetic acid causes hepatocellular tumors in both rats and mice. There is an extensive database on genetic toxicity. A variety of results show that chloral hydrate is a weak gene mutagen and clastogen. Chloral hydrate induces aneuploidy in a wide variety of cell types. These latter effects are thought to arise by disruption of the spindle apparatus. A high concentration of chloral hydrate is required to cause observable effects. Although these data suggest that genotoxicity may play a role in the toxicity of chloral hydrate, the data indicate that these effects require concentrations that are unlikely to occur under physiological conditions at the exposures typically encountered from the environment. Collectively, these data provide suggestive evidence of carcinogenicity, but the weight of evidence is not sufficient to conduct a risk assessment assuming a linear response at low exposure. HUMAN CARCINOGENICITY DATA: None. ANIMAL CARCINOGENICITY DATA: Limited.
Reproductive toxicity
no data available
STOT-single exposure
no data available
STOT-repeated exposure
no data available
Aspiration hazard
no data available
12.Ecological information
12.1 Toxicity
- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: EC50; Species: Daphnia magna (Water flea); Conditions: freshwater; pH 8; Concentration: 630 mg/L for 24 hr; Effect: behavior, equilibrium /Conditions of bioassay not specified in source examined
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
12.2 Persistence and degradability
AEROBIC: Biodegradation data for chloral hydrate were not available(1).
12.3 Bioaccumulative potential
An estimated BCF of 3 was calculated in fish for chloral hydrate(SRC), using a log Kow of 0.99(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low(SRC).
12.4 Mobility in soil
The Koc of chloral hydrate is estimated as 82(SRC), using a log Kow of 0.99(1) and a regression-derived equation(2). According to a classification scheme(3), this estimated Koc value suggests that chloral hydrate is expected to have high mobility in soil.
12.5 Other adverse effects
no data available
13.Disposal considerations
13.1 Disposal methods
Product
The material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packaging
Containers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information
14.1 UN Number
| ADR/RID: UN2811 | IMDG: UN2811 | IATA: UN2811 |
14.2 UN Proper Shipping Name
| ADR/RID: TOXIC SOLID, ORGANIC, N.O.S. |
| IMDG: TOXIC SOLID, ORGANIC, N.O.S. |
| IATA: TOXIC SOLID, ORGANIC, N.O.S. |
14.3 Transport hazard class(es)
| ADR/RID: 6.1 | IMDG: 6.1 | IATA: 6.1 |
14.4 Packing group, if applicable
| ADR/RID: III | IMDG: III | IATA: III |
14.5 Environmental hazards
| ADR/RID: no | IMDG: no | IATA: no |
14.6 Special precautions for user
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Code
no data available
15.Regulatory information
15.1 Safety, health and environmental regulations specific for the product in question
| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| chloral hydrate | chloral hydrate | 302-17-0 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Not Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Not Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
16.Other information
Information on revision
| Creation Date | Aug 11, 2017 |
|---|---|
| Revision Date | Aug 11, 2017 |
Abbreviations and acronyms
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
References
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/








-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
More Suppliers>>Baoji Guokang Bio-Technology Co., Ltd
CHINA
Purity: 98%
Lead Time: 5 Day(s)
Price: Min $28 /kg
Baoji Guokang Bio-Technology Co., Ltd
CHINA
Purity: 99%
Lead Time: 3 Day(s)
Price: -
Wenzhou Win-Win Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Hangzhou J&H Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Henan Coreychem Co.,Ltd
CHINA
Purity: 98%
Lead Time: 3 Day(s)
Price: Min $1 /g
Henan Coreychem Co.,Ltd
CHINA
Purity: 98%
Lead Time: 2-3 Day(s)
Price: -
Shanghai Jizhi Biochemical Technology Co., Ltd.
CHINA
Purity: 99%
Lead Time: 1 Week(s)
Price: -
Skyrun Industrial Co., Limited
CHINA
Purity: 99%
Lead Time: 7 Day(s)
Price: -
Hangzhou Bingochem Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Elsa Biotechnology Co.,Ltd.
CHINA
Purity: %
Lead Time: Day(s)
Price: -