1.Identification
1.1 GHS Product identifier
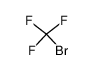
| Product name | BROMOTRIFLUOROMETHANE |
|---|
1.2 Other means of identification
| Product number | - |
|---|---|
| Other names | monobromotrifluoromethane |
1.3 Recommended use of the chemical and restrictions on use
| Identified uses | For industry use only. |
|---|---|
| Uses advised against | no data available |
1.4 Supplier's details
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
1.5 Emergency phone number
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
2.Hazard identification
2.1 Classification of the substance or mixture
Gases under pressure: Liquefied gas
Hazardous to the ozone layer, Category 1
2.2 GHS label elements, including precautionary statements
| Pictogram(s) | 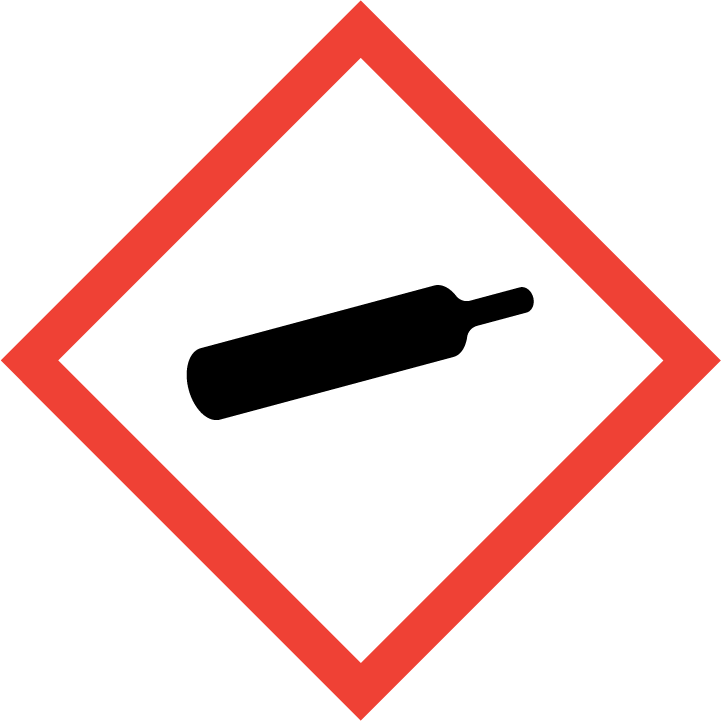  |
|---|---|
| Signal word | Warning |
| Hazard statement(s) | H280 Contains gas under pressure; may explode if heated H420 Harms public health and the environment by destroying ozone in the upper atmosphere |
| Precautionary statement(s) | |
| Prevention | none |
| Response | none |
| Storage | P410+P403 Protect from sunlight. Store in a well-ventilated place. |
| Disposal | P502 Refer to manufacturer or supplier for information on recovery or recycling |
2.3 Other hazards which do not result in classification
none
3.Composition/information on ingredients
3.1 Substances
| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| BROMOTRIFLUOROMETHANE | BROMOTRIFLUOROMETHANE | 75-63-8 | none | 100% |
4.First-aid measures
4.1 Description of necessary first-aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
Fresh air, rest. Artificial respiration may be needed. Refer for medical attention.
In case of skin contact
ON FROSTBITE: rinse with plenty of water, do NOT remove clothes. Refer for medical attention .
In case of eye contact
First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
4.2 Most important symptoms/effects, acute and delayed
Excerpt from ERG Guide 126 [Gases - Compressed or Liquefied (Including Refrigerant Gases)]: Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases. (ERG, 2016)
4.3 Indication of immediate medical attention and special treatment needed, if necessary
If the diagnosis of solvent abuse is suspected it can be confirmed by biochemical examination of the blood or urine. Emergency treatment is supportive and includes decontamination, oxygen, and any specific therapy required in a particular case such as antiarrhythmics or anticonvulsants. A few patients may require intermittent positive-pressure ventilation, dialysis, or treatment for hepatic failure. /Solvent abuse/
5.Fire-fighting measures
5.1 Extinguishing media
Suitable extinguishing media
If material involved in fire: Extingiush fire using agent suitable for type of surrounding fire. (Material itself does not burn or burns with difficulty). Cool all affected containers with flooding quantities of water. Apply water from as far a distance as possible.
5.2 Specific hazards arising from the chemical
Excerpt from ERG Guide 126 [Gases - Compressed or Liquefied (Including Refrigerant Gases)]: Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket. (ERG, 2016)
5.3 Special protective actions for fire-fighters
Wear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures
6.1 Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautions
Ventilation. NEVER direct water jet on liquid. Personal protection: chemical protection suit including self-contained breathing apparatus.
6.3 Methods and materials for containment and cleaning up
If trifluoromonobromomethane is leaked, the following steps should be taken: 1. Ventilate area of leak. 2. Stop flow of gas.
7.Handling and storage
7.1 Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Fireproof if in building. Cool.
8.Exposure controls/personal protection
8.1 Control parameters
Occupational Exposure limit values
Recommended Exposure Limit: 10 Hr Time-Weighted Avg: 1000 ppm (6100 mg/cu m)
Biological limit values
no data available
8.2 Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE)
Eye/face protection
Safety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Wear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protection
Wear dust mask when handling large quantities.
Thermal hazards
no data available
9.Physical and chemical properties
| Physical state | Bromotrifluoromethane is a colorless, odorless gas at room conditions Shipped as a liquid confined under its own vapor pressure. Noncombustible. Nontoxic but can asphyxiate by the displacement of air. Contact with the unconfined liquid can cause frostbite by evaporative cooling. Exposure of the container to prolonged heat or fire can cause it to rupture violently and rocket. |
|---|---|
| Colour | Colorless gas [Note: Shipped as a liquefied compressed gas.] |
| Odour | Odorless gas. |
| Melting point/ freezing point | -168ºC |
| Boiling point or initial boiling point and boiling range | -58ºC |
| Flammability | Nonflammable GasNot combustible. Heating will cause rise in pressure with risk of bursting. Gives off irritating or toxic fumes (or gases) in a fire. |
| Lower and upper explosion limit / flammability limit | no data available |
| Flash point | no data available |
| Auto-ignition temperature | no data available |
| Decomposition temperature | no data available |
| pH | no data available |
| Kinematic viscosity | 0.157 mPa.s @ 25°C (liq); 0.0154 mPa.s @ 25°C - 101.3 kPa (vapor) |
| Solubility | 0.03 % (NIOSH, 2016) |
| Partition coefficient n-octanol/water (log value) | log Kow= 1.86 |
| Vapour pressure | greater than 1 atm (NIOSH, 2016) |
| Density and/or relative density | 1.58 |
| Relative vapour density | 3.8 (AIR= 1) |
| Particle characteristics | no data available |
10.Stability and reactivity
10.1 Reactivity
no data available
10.2 Chemical stability
Conditions contributing to instability: heat
10.3 Possibility of hazardous reactions
MONOBROMOTRIFLUOROMETHANE IS NOT FLAMMABLE; IT IS A GOOD FIRE EXTINGUISHER.The vapour is heavier than air and may accumulate in lowered spaces causing a deficiency of oxygen.BROMOTRIFLUOROMETHANE may react with aluminum to produce substantial heat. Other halogenated hydrocarbons, such as fluorotrichloromethane, dichlorodifluoromethane, chlorodifluoromethane, tetrafluoromethane produce sufficient heat in this way to melt aluminum pieces. The vigor of the reaction appears to depend on the degree of fluorination and the vapor pressure [Chem. Eng. News 39(27):44 1961].
10.4 Conditions to avoid
no data available
10.5 Incompatible materials
Incompatible with chemically-active metals such as calcium, powdered aluminum, zinc & magnesium.
10.6 Hazardous decomposition products
THE FIRE EXTINGUISHER HALON 1301 BEGINS TO DECOMP AT 400-500 DEG TO HALOGEN GASES, WHICH REACT WITH HYDROGEN TO FORM HYDROGEN HALIDES. IN OXYGEN, CARBON DIOXIDE, CARBONYL FLUORIDE & CARBONYL BROMIDE MAY FORM. HAZARDS FROM DECOMP PRODUCTS ARE NEGLIGIBLE AS COMPARED TO THOSE OF OTHER HAZARDS ASSOCIATED WITH A FIRE.
11.Toxicological information
Acute toxicity
- Oral: no data available
- Inhalation: no data available
- Dermal: no data available
Skin corrosion/irritation
no data available
Serious eye damage/irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
no data available
Reproductive toxicity
no data available
STOT-single exposure
no data available
STOT-repeated exposure
no data available
Aspiration hazard
no data available
12.Ecological information
12.1 Toxicity
- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
12.2 Persistence and degradability
Based upon the highly halogenated structure of bromotrifluoromethane, biodegradation is expected to be slow(1).
12.3 Bioaccumulative potential
An estimated BCF of 5.4 was calculated for bromotrifluoromethane(SRC), using a log Kow of 1.86(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low.
12.4 Mobility in soil
The Koc of bromotrifluoromethane is estimated as 49(SRC), using a log Kow of 1.86(1) and a regression-derived equation(2). According to a classification scheme(3), this estimated Koc value suggests that bromotrifluoromethane is expected to have very high mobility in soil.
12.5 Other adverse effects
no data available
13.Disposal considerations
13.1 Disposal methods
Product
The material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packaging
Containers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information
14.1 UN Number
| ADR/RID: UN1009 | IMDG: UN1009 | IATA: UN1009 |
14.2 UN Proper Shipping Name
| ADR/RID: BROMOTRIFLUOROMETHANE (REFRIGERANT GAS R 13B1) |
| IMDG: BROMOTRIFLUOROMETHANE (REFRIGERANT GAS R 13B1) |
| IATA: BROMOTRIFLUOROMETHANE (REFRIGERANT GAS R 13B1) |
14.3 Transport hazard class(es)
| ADR/RID: 2.2 | IMDG: 2.2 | IATA: 2.2 |
14.4 Packing group, if applicable
| ADR/RID: unknown | IMDG: unknown | IATA: unknown |
14.5 Environmental hazards
| ADR/RID: no | IMDG: no | IATA: no |
14.6 Special precautions for user
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Code
no data available
15.Regulatory information
15.1 Safety, health and environmental regulations specific for the product in question
| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| BROMOTRIFLUOROMETHANE | BROMOTRIFLUOROMETHANE | 75-63-8 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Not Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
16.Other information
Information on revision
| Creation Date | Aug 17, 2017 |
|---|---|
| Revision Date | Aug 17, 2017 |
Abbreviations and acronyms
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
References
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/










-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
More Suppliers>>Hangzhou J&H Chemical Co., Ltd.
CHINA
Purity: >97%
Lead Time: 10 Day(s)
Price: -
Hangzhou DayangChem Co., Ltd
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Skyrun Industrial Co., Limited
CHINA
Purity: 99%
Lead Time: 7 Day(s)
Price: -
Zhejiang Chemical Industry Technology Co., Ltd.
CHINA
Purity: ≥ 99.6%
Lead Time: 15 Day(s)
Price: -
Jinan Fluorine Chemical Co., Ltd.
CHINA
Purity: 99.6%
Lead Time: 14 Day(s)
Price: -
Beijing Coupling Technology Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: Min $46.67 /ml
Shanghai qinba chemical co tdl
CHINA
Purity: 98%
Lead Time: 3 Day(s)
Price: -
Hangzhou Hete Chemical Technology., Ltd.
CHINA
Purity: 98%
Lead Time: 14 Day(s)
Price: -
Beijing Huaruisheng Technology Co., Ltd.
CHINA
Purity: 99.99%
Lead Time: 14 Day(s)
Price: -