1.Identification
1.1 GHS Product identifier
| Product name | Ammonium Perchlorate |
|---|
1.2 Other means of identification
| Product number | - |
|---|---|
| Other names | azanium perchlorate |
1.3 Recommended use of the chemical and restrictions on use
| Identified uses | For industry use only. Oxidizing/reducing agents |
|---|---|
| Uses advised against | no data available |
1.4 Supplier's details
| Company | MOLBASE (Shanghai) Biotechnology Co., Ltd. |
|---|---|
| Address | Floor 4 & 5, Building 12, No. 1001 North Qinzhou Road, Xuhui District, Shanghai, China |
| Telephone | +86(21)64956998 |
| Fax | +86(21)54365166 |
1.5 Emergency phone number
| Emergency phone number | +86-400-6021-666 |
|---|---|
| Service hours | Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). |
2.Hazard identification
2.1 Classification of the substance or mixture
Explosives, Division 1.1
Oxidizing solids, Category 1
2.2 GHS label elements, including precautionary statements
| Pictogram(s) | 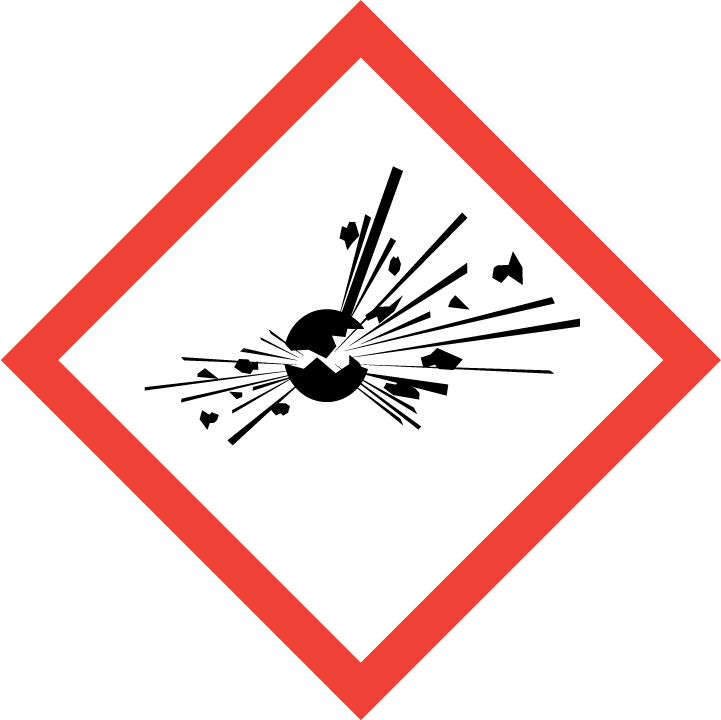 |
|---|---|
| Signal word | Danger |
| Hazard statement(s) | H201 Explosive; mass explosion hazard H271 May cause fire or explosion; strong oxidizer |
| Precautionary statement(s) | |
| Prevention | P210 Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P230 Keep wetted with … P234 Keep only in original packaging. P240 Ground and bond container and receiving equipment. P250 Do not subject to grinding/shock/friction/…. P280 Wear protective gloves/protective clothing/eye protection/face protection. P220 Keep away from clothing and other combustible materials. P283 Wear fire resistant or flame retardant clothing. |
| Response | P370+P372+P380+P373 In case of fire: Explosion risk. Evacuate area. DO NOT fight fire when fire reaches explosives. P306+P360 IF ON CLOTHING: Rinse immediately contaminated clothing and skin with plenty of water before removing clothes. P371+P380+P375 In case of major fire and large quantities: Evacuate area. Fight fire remotely due to the risk of explosion. P370+P378 In case of fire: Use ... to extinguish. |
| Storage | P401 Store in accordance with… P420 Store separately. |
| Disposal | P501 Dispose of contents/container to ... |
2.3 Other hazards which do not result in classification
none
3.Composition/information on ingredients
3.1 Substances
| Chemical name | Common names and synonyms | CAS number | EC number | Concentration |
|---|---|---|---|---|
| Ammonium Perchlorate | Ammonium Perchlorate | 7790-98-9 | none | 100% |
4.First-aid measures
4.1 Description of necessary first-aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
Fresh air, rest.
In case of skin contact
First rinse with plenty of water for at least 15 minutes, then remove contaminated clothes and rinse again.
In case of eye contact
First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention.
If swallowed
Rinse mouth. Give one or two glasses of water to drink.
4.2 Most important symptoms/effects, acute and delayed
Irritating to skin and mucous membranes. (USCG, 1999)
4.3 Indication of immediate medical attention and special treatment needed, if necessary
Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for signs of pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mg/kg up to 200 ml of water for dilution if the patent can swallow, has a strong gag reflex, and does not drool ... . Do not attempt to neutralize. /Ammonia and related compounds/
5.Fire-fighting measures
5.1 Extinguishing media
Suitable extinguishing media
If material on fire or involved in fire: Dangerously explosive. Do not fight fires in a cargo of explosives. Evacuate area and let burn. /Explosives/
5.2 Specific hazards arising from the chemical
Special Hazards of Combustion Products: Toxic gases are produced in a fire. Behavior in Fire: May explode when involved in fire or exposed to shock or friction. (USCG, 1999)
5.3 Special protective actions for fire-fighters
Wear self-contained breathing apparatus for firefighting if necessary.
6.Accidental release measures
6.1 Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. For personal protection see section 8.
6.2 Environmental precautions
Evacuate danger area! Consult an expert! Personal protection: particulate filter respirator adapted to the airborne concentration of the substance. Do NOT let this chemical enter the environment. If appropriate, moisten first to prevent dusting. Sweep spilled substance into covered containers. Then store and dispose of according to local regulations. Do NOT absorb in saw-dust or other combustible absorbents.
6.3 Methods and materials for containment and cleaning up
Ion exchange is an ex situ technology used to remove perchlorate from drinking water, groundwater, surface water, and environmental media at full scale. ... The most commonly used ion exchange media are synthetic, strongly basic, anion exchange resins. Ion exchange has been used at sites to reduce perchlorate concentrations to less than 4 ug/L. Its effectiveness is sensitive to a variety of untreated water contaminants and characteristics. It has also been used as a polishing step for other water treatment processes such as biological treatment of perchlorate. Ion exchange of perchlorate in environmental media and drinking water is commercially available. Information is available on 15 full-scale applications, including 11 applications for environmental media, and four applications for drinking water. Three pilot-scale applications for groundwater also have been identified. ...For the 14 groundwater projects (11 full scale and three pilot scale), influent perchlorate concentrations ranged from 10 ug/L to 350,000 ug/L. Effluent concentrations of perchlorate ranged from non-detectable at a detection limit of 0.35 ug/L to 2,000 ug/L. Of the four drinking water projects, performance data were available for only one project. The initial concentration of perchlorate in this project ranged from 20 to 50 ug/L, while the final concentration was below the detection limit of 4 ug/L. ...Cleanup goals varied by site and type of project. /Perchlorates/
7.Handling and storage
7.1 Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure - obtain special instructions before use.Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2.
7.2 Conditions for safe storage, including any incompatibilities
Fireproof. Separated from combustible substances, reducing agents and metals. See Chemical Dangers. Well closed.Separate from acids, alkalies reducing agents, combustible materials. Store in cool, dry, well-ventilated location.
8.Exposure controls/personal protection
8.1 Control parameters
Occupational Exposure limit values
no data available
Biological limit values
no data available
8.2 Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.
8.3 Individual protection measures, such as personal protective equipment (PPE)
Eye/face protection
Safety glasses with side-shields conforming to EN166. Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Wear impervious clothing. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique(without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it.
Respiratory protection
Wear dust mask when handling large quantities.
Thermal hazards
no data available
9.Physical and chemical properties
| Physical state | crystalline solid |
|---|---|
| Colour | Colorless, crystalline compound |
| Odour | No odor |
| Melting point/ freezing point | °Cd ec.) |
| Boiling point or initial boiling point and boiling range | no data available |
| Flammability | Not combustible but enhances combustion of other substances. Many reactions may cause fire or explosion. Gives off irritating or toxic fumes (or gases) in a fire. See Notes. |
| Lower and upper explosion limit / flammability limit | no data available |
| Flash point | no data available |
| Auto-ignition temperature | 240°C (USCG, 1999) |
| Decomposition temperature | >200°C |
| pH | no data available |
| Kinematic viscosity | no data available |
| Solubility | In water, 2.0X10+5 mg/L at 25°C |
| Partition coefficient n-octanol/water (log value) | no data available |
| Vapour pressure | no data available |
| Density and/or relative density | 1.95 |
| Relative vapour density | no data available |
| Particle characteristics | no data available |
10.Stability and reactivity
10.1 Reactivity
no data available
10.2 Chemical stability
Stable under recommended storage conditions.
10.3 Possibility of hazardous reactions
IGNITES VIOLENTLY WITH COMBUSTIBLES.AMMONIUM PERCHLORATE is a strong oxidizing agent. Decomposes at 130°C and explodes at 380°C [Mellor 2 Supp. 1:608 1956]. Explosions have occurred in propellant formulations containing ammonium perchlorate to which ferrocene has been added as a burning rate catalyst. Although the cause was not been definitely established, it was most probably frictional heating from dragging a spatula through the mixture [ASESB Expl. Report 211 1966]. Can explode when mixed with sugar, charcoal or on contact with hot copper pipes. Becomes impact-sensitive when contaminated by powdered carbon, ferrocene, sulfur, or other reducing materials such as organic matter or powdered metals.
10.4 Conditions to avoid
no data available
10.5 Incompatible materials
Ammonium perchlorate decomposes at 130 DEG C and explodes at 380 DEG C
10.6 Hazardous decomposition products
Oxides of nitrogen (except nitrous oxide), hydrogen chloride, and ammonia /are/ emitted on decomposition of ammonium perchlorate...
11.Toxicological information
Acute toxicity
- Oral: LD50 Rat oral 4200 mg/kg
- Inhalation: no data available
- Dermal: no data available
Skin corrosion/irritation
no data available
Serious eye damage/irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
no data available
Reproductive toxicity
no data available
STOT-single exposure
no data available
STOT-repeated exposure
no data available
Aspiration hazard
no data available
12.Ecological information
12.1 Toxicity
- Toxicity to fish: no data available
- Toxicity to daphnia and other aquatic invertebrates: no data available
- Toxicity to algae: no data available
- Toxicity to microorganisms: no data available
12.2 Persistence and degradability
ANAEROBIC: Microorganisms isolated from soil have been found to reduce perchlorates under anaerobic conditions using laboratory tests(1). Perchlorate applied to Yolo loam at a concentration of 180 mg/L and incubated anaerobically under flooded conditions was completely biodegraded after 30 days(1). No loss was observed using a Columbia loam soil(1). The facultative anaerobes belonging to the genera Riemerella, Acidovorax and Azoarcus together may be capable of perchlorate reduction(2). However, nitrate does interfere with perchlorate reduction(3). Using sediment and soil samples obtained from two Texas sites associated historically with perchlorate discharge, anaerobic microcosms studies indicate that rapid perclorate degradation did not occur until nitrate was degraded to a relatively low level(3).
12.3 Bioaccumulative potential
Using a plant-mediated treatment of perchlorate-contaminated water, perchlorate uptake occurred in eastern cottonwoods (Populus deltoides and hybrid populus), Eucalyptus cineria, and willow (Salix nigra) in sand bioreactors. Perchlorate uptake in willows was found initially rapid at a rate that was linear with the volume of water evapotranspired by the tree until a plateau was reached. From an initial application of 88.8 mg (96.4 mg/L), the total amounts of perchlorate in root, lower stem, upper stem, and leaf after 26 days were 0.04, 0.18, 0.34 and 0.48 mg, respectively. 11% of the perchlorate was not accounted for and believed to be degraded in the leaves(1).
12.4 Mobility in soil
Ammonium perchlorate readily dissolves and dissociates to the perchlorate ion(1). The perchlorate ion is only weakly absorbed to mineral surfaces of moderate ionic strength(1). The ion exhibits high aqueous solubility and together these properties contribute to its ability to readily migrate in groundwater systems(2).
12.5 Other adverse effects
no data available
13.Disposal considerations
13.1 Disposal methods
Product
The material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.
Contaminated packaging
Containers can be triply rinsed (or equivalent) and offered for recycling or reconditioning. Alternatively, the packaging can be punctured to make it unusable for other purposes and then be disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possible for combustible packaging materials.
14.Transport information
14.1 UN Number
| ADR/RID: UN1442 | IMDG: UN1442 | IATA: UN1442 |
14.2 UN Proper Shipping Name
| ADR/RID: AMMONIUM PERCHLORATE |
| IMDG: AMMONIUM PERCHLORATE |
| IATA: AMMONIUM PERCHLORATE |
14.3 Transport hazard class(es)
| ADR/RID: 5.1 | IMDG: 5.1 | IATA: 5.1 |
14.4 Packing group, if applicable
| ADR/RID: II | IMDG: II | IATA: II |
14.5 Environmental hazards
| ADR/RID: no | IMDG: no | IATA: no |
14.6 Special precautions for user
no data available
14.7 Transport in bulk according to Annex II of MARPOL 73/78 and the IBC Code
no data available
15.Regulatory information
15.1 Safety, health and environmental regulations specific for the product in question
| Chemical name | Common names and synonyms | CAS number | EC number |
|---|---|---|---|
| Ammonium Perchlorate | Ammonium Perchlorate | 7790-98-9 | none |
| European Inventory of Existing Commercial Chemical Substances (EINECS) | Listed. | ||
| EC Inventory | Listed. | ||
| United States Toxic Substances Control Act (TSCA) Inventory | Listed. | ||
| China Catalog of Hazardous chemicals 2015 | Listed. | ||
| New Zealand Inventory of Chemicals (NZIoC) | Listed. | ||
| Philippines Inventory of Chemicals and Chemical Substances (PICCS) | Listed. | ||
| Vietnam National Chemical Inventory | Not Listed. | ||
| Chinese Chemical Inventory of Existing Chemical Substances (China IECSC) | Listed. | ||
16.Other information
Information on revision
| Creation Date | Aug 12, 2017 |
|---|---|
| Revision Date | Aug 12, 2017 |
Abbreviations and acronyms
- CAS: Chemical Abstracts Service
- ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road
- RID: Regulation concerning the International Carriage of Dangerous Goods by Rail
- IMDG: International Maritime Dangerous Goods
- IATA: International Air Transportation Association
- TWA: Time Weighted Average
- STEL: Short term exposure limit
- LC50: Lethal Concentration 50%
- LD50: Lethal Dose 50%
- EC50: Effective Concentration 50%
References
- IPCS - The International Chemical Safety Cards (ICSC), website: http://www.ilo.org/dyn/icsc/showcard.home
- HSDB - Hazardous Substances Data Bank, website: https://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
- IARC - International Agency for Research on Cancer, website: http://www.iarc.fr/
- eChemPortal - The Global Portal to Information on Chemical Substances by OECD, website: http://www.echemportal.org/echemportal/index?pageID=0&request_locale=en
- CAMEO Chemicals, website: http://cameochemicals.noaa.gov/search/simple
- ChemIDplus, website: http://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
- ERG - Emergency Response Guidebook by U.S. Department of Transportation, website: http://www.phmsa.dot.gov/hazmat/library/erg
- Germany GESTIS-database on hazard substance, website: http://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp
- ECHA - European Chemicals Agency, website: https://echa.europa.eu/